Request Kits
Test kits are available upon request to providers with a US BioTek account. If you do not have a US BioTek account you can create one here.
Our 295 Food & Inhalant Panel provide a detailed assessment of whole extract and specific IgE components.
New IgE panels measure whole allergen extracts and over 50 molecular allergens providing a more complete clinical picture of IgE allergies and IgE cross-reactions.
The microtainer blood collection method allows for convenient, self-administered blood collection.
Since the structure and cross-reactivity of specific IgE (sIgE) components are known, the use of our new 295 Food, Inhalant & Components IgE Panel, 158 IgE Food/Components Panel, and 140 IgE Inhalant/Components Panel can help unravel cross-reactivity in complex, multi-sensitized patients. While there are multiple forms of treatment for IgE allergies, food avoidance and allergy immunotherapy (AIT) are front-line therapies that can be supported by the 295 IgE profile and its unique blend of whole extract allergens and specific IgE components.
The simultaneous measurement of whole allergen extracts and over 50 molecular allergens (“specific IgE” or sIgE) provides a more complete clinical picture of not only the patient’s IgE allergies but IgE cross-reactions. sIgE results also guide the selection of antigens for allergy immunotherapy (AIT).
The inhibition of cross-reactive carbohydrate determinants (CCDs) reactions. CCDs are known confounders that produce a “false positive” IgE result in up to 20% of patients. Patients with high CCD-driven results needlessly eliminate foods from their diets.
State of the art multiplex ELISA testing using nanobead technology validated in multiple human studies.
While there are multiple forms of treatment for IgE allergies, food avoidance and allergy immunotherapy (AIT) are front-line therapies that can be supported by the 295 IgE profile and its unique blend of whole extract allergens and specific IgE components.
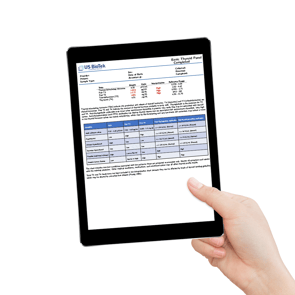
Our reports are well-recognized for their clear visual representation of results, providing an easy-to-read, easy-to-understand foundation from which healthcare providers, together with their patients, can establish a plan for improved health.
Our IgE panels utilize a quantitative ELISA (enzyme linked immunosorbant assay) analysis of the specific immunoglobin IgE and IgG4 identified for the chosen diet panels’ food and spice analytes.
We follow a meticulous process to extract the critical component from each analyte (food and inhalant trigger) to ensure the antibody present in the patients’ sample will bind properly and validate results with positive and negative controls. Once attached to the antigens, antibodies are detected through spectrophotometric analysis, where the values are directly proportional to the concentration of the analytes in the sample. For additional accuracy, we perform duplicate testing to ensure there are no discrepancies.

We provide an easy-to-read, easy-to-understand foundation from which healthcare providers, together with their patients, can establish a plan for improved health.
See for yourself. Below are sample reports for all of our test options.
![]()
The serum collection method requires a modest blood draw.
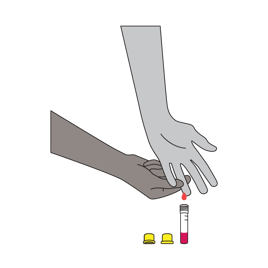
The microtainer blood collection method allows for convenient, self-administered blood collection.
View Collection Instructions>>
Be prepared for the new IgE Food and Inhalant panels by ordering your free test kits.
Test kits are available upon request to providers with a US BioTek account. If you do not have a US BioTek account you can create one here.
We invite healthcare providers to set up an account with us. Our services can be ordered by a healthcare provider who is authorized under his or her scope of practice to order tests and interpret results for the purposes of diagnosis and decisions regarding patient treatment.
Providers can view and download results through our secure Clinician Portal, or request results by email.
Please note that US BioTek does not discuss test results directly with patients. Practitioners assume the responsibility of relaying test results to their patients. All results are confidential.
Test kits are available to registered providers upon request. Use our online submission form to order the collection kits you require.
Easy collection and low volume specimen requirements are advantages of the US BioTek testing methodology. Follow our simple instructions.
Upon completion of specimen collection, complete our test requisition form and include it in your specimen shipment.
Providers can view and download results through our secure Clinician Portal, or request results by email.
Please note that US BioTek does not discuss test results directly with patients. Practitioners assume the responsibility of relaying test results to their patients. All results are confidential.